Parler
Parler Gab
Gab
Other countries have permanently dropped AstraZeneca vaccine
While Italy limited its use of the AstraZeneca vaccine, other nations have removed it from their respective COVID-19 vaccination programs. Health authorities in Denmark, where the first cases of blood clots following AstraZeneca vaccination were reported, initially suspended the vaccine in March 2021. A month later, the Danish Health Authority (SST) announced that COVID-19 vaccinations will continue without the AstraZeneca vaccine. In an April 14 statement, SST Director General Søren Brostrøm said: "Based on the scientific findings, our overall assessment is [that] there is a real risk of severe side effects associated with using the COVID-19 vaccine from AstraZeneca. We have, therefore, decided to remove the vaccine from our vaccination program. He added that permanently halting the use of AstraZeneca's COVID-19 vaccine "has been a difficult decision." But Brostrøm nevertheless continued that Denmark "[had] other vaccines at [its] disposal" such as the Pfizer/BioNTech and Moderna vaccines. (Related: Denmark permanently stops rollout for AstraZeneca vaccine, citing concerns about blood clots.) Meanwhile, Norway also followed the footsteps of its southern neighbor and suspended the AstraZeneca vaccine. According to a Bloomberg report, Norwegian Prime Minister Erna Solberg announced the suspension during a May 12 news conference. "The government has decided that the AstraZeneca vaccine will not be used in Norway, not even voluntarily," she told reporters. Solberg's advice followed a recommendation by the Norwegian Institute of Public Health (NIPH) to suspend the vaccine's use in Norway's COVID-19 inoculation program. NIPH Infection Control and Environmental Health Division Director Geir Bukholm said on April 15: "We now know significantly more about the connection between the AstraZeneca vaccine and the rare and serious incidents of low platelets, blood clots and bleeding. Based on this knowledge, we have arrived at a recommendation that the AstraZeneca vaccine be removed from the coronary vaccination program in Norway." (Related: Norwegian health agency recommends banning AstraZeneca coronavirus vaccine due to blood clot risks.) Visit VaccineDamage.news to read more about the dangers of the AstraZeneca COVID-19 vaccine. Sources include: TheEpochTimes.com Reuters.com SST.dk Bloomberg.com FHI.noDeborah Birx hid covid info from Trump, altered CDC guidelines without approval
By Ethan Huff // Share
Germany’s birth rate improbably falls by 11% in the first quarter of 2022
By Lance D Johnson // Share
By Mary Villareal // Share
Common signs and symptoms of magnesium deficiency
By Olivia Cook // Share
Arugula rocks your nutrition world
By newseditors // Share
Oil prices crash -- Trump seizes opportunity to rebuild depleted SPR
By willowt // Share
Betrayal: Trump bets on Argentine beef as domestic producers reel
By willowt // Share
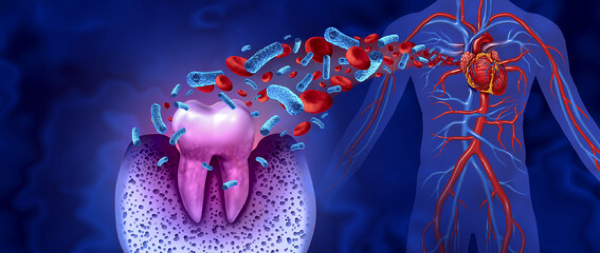
The hidden heart risk in your mouth: How gum disease clogs arteries
By willowt // Share