Parler
Parler Gab
Gab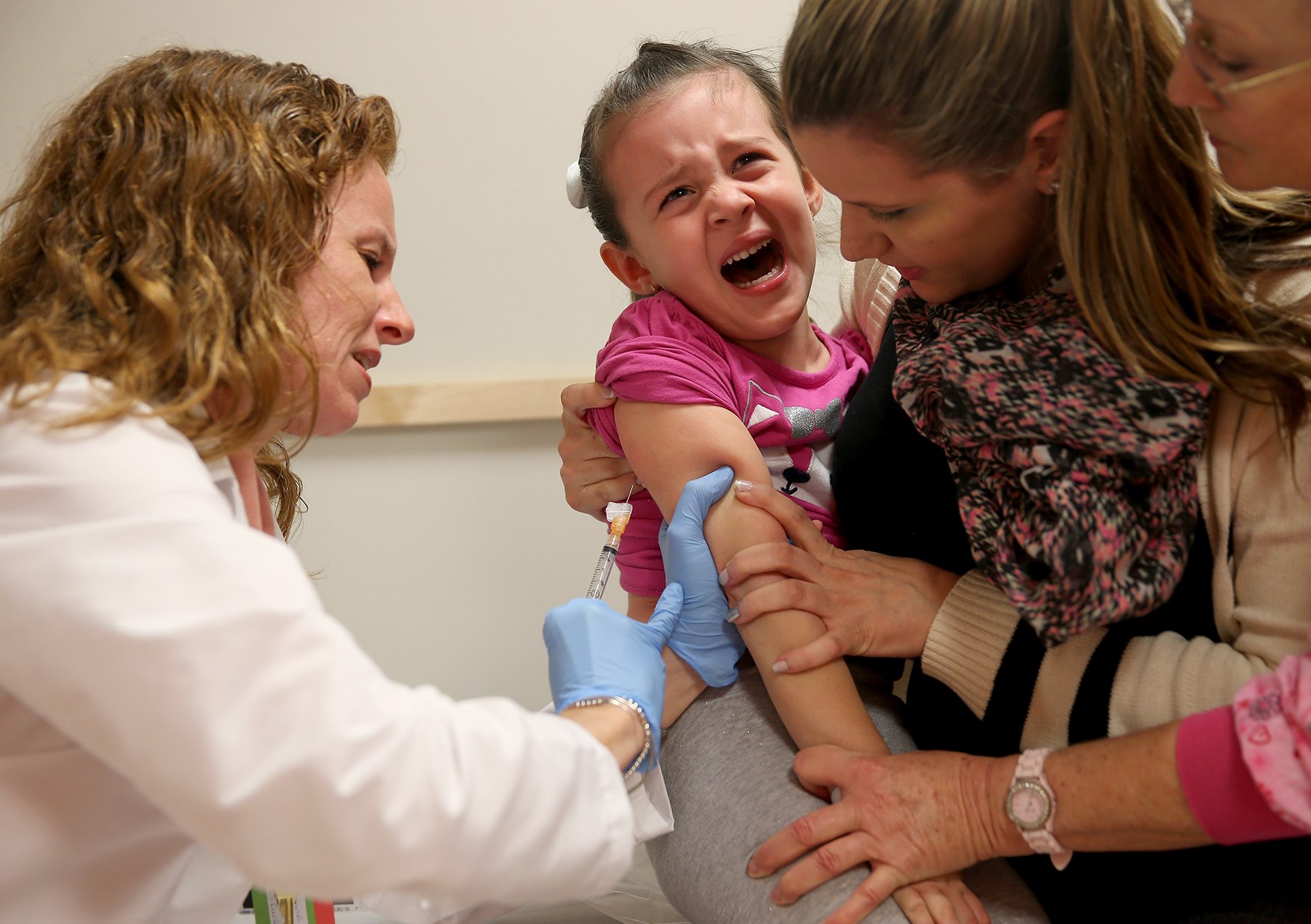
Pfizer risking the health of young children for profit
Regardless of the many risks associated with coronavirus vaccines, mandatory vaccination programs are often quickly rolled out in many countries, As of writing, millions of doses of experimental vaccines have been administered while researchers have yet to determine the full extent of vaccine-related health risks to people of various ages. Even 12-year-old children are now allowed to get vaccinated because of Pfizer’s efforts to earn EUA for the age group. But Dr. Yaffa Shir-Raz, a researcher from Israel, warned that Pfizer violated its own protocol to ensure that its vaccine can be administered to young children. On June 8, Shir-Raz, a researcher and fellow at the University of Haifa and Interdisciplinary Center Herzliya in Israel, wrote an article detailing how Pfizer violated the company's own protocols. The article was published on America’s Frontline Doctors' website. In the article, Shir-Raz explained that Pfizer allowed children with pre-existing psychiatric conditions to be enrolled in the trial despite the fact that this was listed as exclusion criteria in the company's documentation. According to the review document Pfizer submitted to the FDA, four of the 1,131 children in the study given the Pfizer-BioNTech coronavirus vaccine experienced serious adverse events (SAEs) or events wherein "at least one criterion was met: caused death, is life-threatening, requires hospitalization or prolongation of existing hospitalization, results in persistent disability/incapacity, or congenital anomaly/birth defect." Because of Pfizer's decision, three children (out of 1,131) ended up requiring hospitalization for severe depression within one to 15 days of inoculation:- One child in the first seven days after the first dose
- Another child in the second only one day after the second dose
- And the last child in the third 15 days after the first dose
Pfizer and Big Pharma must be held accountable for these manipulative tactics
But that's not all. Shir-Raz added that Pfizer used other questionable tactics for the trial.- The trial only had a six-month follow-up in its entirety, which is less than the recommended one to four years of follow-up required by the FDA.
- Because of how the protocol was designed, Pfizer only needed to report serious adverse events occurring within the first month after inoculation to the FDA. This means adverse events occurring later wouldn't be considered in the FDA’s safety analysis and that Pfizer's findings would seem more positive than they actually are.
- Pfizer investigators were allowed to define the adverse events for themselves ("As elicited by investigative site staff") instead of relying on any diagnosis from medical professionals at a hospital.
Deborah Birx hid covid info from Trump, altered CDC guidelines without approval
By Ethan Huff // Share
Germany’s birth rate improbably falls by 11% in the first quarter of 2022
By Lance D Johnson // Share
By Mary Villareal // Share
Common signs and symptoms of magnesium deficiency
By Olivia Cook // Share